What’s the Difference Between Calibration and Validation in the Lab?
If you work in a lab, whether it be biotech, pharma, R&D, or clinical, you likely know of the need for calibration and validation of your equipment and processes. You also likely hear some of your colleagues use the words interchangeably.
Although they may sound similar, and the processes are related, they should not be confused with one another.
Calibration of lab equipment and sensors is a process through which a measured value is compared against a calibrated standard to determine the measured value’s accuracy. Some manufacturers test and calibrate equipment on the assembly line and provide a calibration certificate. This certificate only has value if the sensors used can be traceable to, for example, Raad voor Accreditatie (RvA), UKAS or NIST. Without traceability, it is hard to declare a device fit for purpose, because a display value cannot be trusted fully.
Even if a device or sensor is calibrated, this does not automatically mean a piece of equipment or room is fit for purpose. The measured value of a sensor can be absolutely spot on, but it tells nothing about the behaviour of a room or piece of equipment in case of different loads, power failure, or door opening. Before a piece of equipment can be declared fit for purpose, the process of validation is meant to ensure that a room or device is capable of performing according to the specifications of the samples meant to be stored in it.
Let’s dig into each in a little more detail.
Calibration of Equipment: Accuracy For Your Lab
It’s likely that the scale in your home bathroom is probably off by a pound or two and in need of calibration, but this is probably not keeping you up at night.
In the lab, improperly calibrated equipment is outright dangerous. If samples are stored in a room or device with an uncalibrated sensor, users are likely to make decisions based on incorrect information. If, for example, blood is kept in a fridge showing the wrong temperature, users might think they are doing everything correctly, whilst the blood is incorrectly stored. Giving this blood to the patient later can put this patient’s life at risk. The same goes for medicines or food.
Calibration is also required for every quality management system, such as ISO, FDA, CAP, CLIA, and GMP. Showing your equipment was and is properly calibrated to meet applicable standards and regulations is critical to keeping a lab moving forward.
While many labs elect to send out their equipment or sensors to be calibrated, this opens up many issues regarding its traceability. How can you be certain a factory calibration certificate holds any value? The rule of thumb is, without NIST traceability, a factory calibration certificate holds no official value.
Depending on the level of accreditation, there are two options. If the accreditation allows, users can calibrate their own devices with a (RvA) calibrated sensor. This allows a good level of control and has a basic traceability of the values. One has to bear in mind that a correct calibration process is not easy, and there is no oversight on the processes used. This means the uncertainty of this process is unknown. In other words, if a user incorrectly calibrates the sensors, they can make things worse.
The second option is to hire a professional RvA traceable calibration company. Their staff are trained in using the correct procedure and should not make any mistakes during the process. The calibration reports will have uncertainty making this kind of calibration service very valuable for laboratories with high accreditation standards. The downside of it is the added costs of hiring a calibration company.
Regardless of who is doing the calibration, the steps are always the same. First, one must create a stable environment where both sensor and standard can be compared. If one deviates from the other, an offset of the sensor can be necessary. After offsetting the sensor value, it is imperative to repeat the calibration process to prove the offset has correctly adjusted the sensor display readout.

Placing sensors in a block oven is a good example of creating a stable environment to calibrate sensors in

When sensors cannot be easily removed placing an aluminium block inside a device will greatly improve stability of the measurements, resulting in a lower uncertainty
Finally, the calibration has to be reported properly. Without written proof, any calibration will hold no value. A user will have to correctly write exactly which steps were taken and which standards were used to perform a calibration. They will also have to show what values were recorded during the calibration process in order to correctly report any offsets performed.
Validation: How to Prove Something is Fit For Purpose
In almost every case, demonstrating that equipment is properly calibrated is a prerequisite to being able to perform validation. Still, validation is a much broader topic; it also consists of: workflows, staff performance, data quality, and more.
Unlike equipment calibration processes specific to each piece of equipment (no matter where it may be in use), validation processes are often unique to each lab and its business model.
Whereas, say, with calibrating a simple scale, an ounce is an ounce, no matter where or how the scale is used. There is no “one size fits all” approach for overall lab validation. It requires a dedicated, business-specific approach and outputs (in case of an issue or an audit wherein a paper trail may be required).
Validation processes for an accredited laboratory regarding sensors used in XiltriX consist of two major parts. Firstly one has to prove equipment and rooms perform according to the specifications for samples to be stored or medicines to be produced. The catch is that while the validation process is often unique, there are still some standards to which all labs must adhere. To cite just one example, the IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) protocols, typically apply to equipment and software systems.
Briefly, they’re defined as follows:
- IQ validates that equipment is correctly installed as per the manufacturer’s instructions (which, naturally, would usually include equipment calibrated as per those instructions as well).
- OQ validates that equipment operates as intended within a range of operations specified by the manufacturer or dictated by a compliance standard.
- PQ integrates usage into the protocols: is your equipment staying in line with standards as it’s being used?
The OQ and PQ parts are the most work. In the example of a blood fridge, being able to trust the display value of the device is the first step of the validation process. Before placing it into the lab, one must know if the device can recover quickly enough after a door opening. Also, the temperature spread across the sample storage inside the fridge is important. This might be different with different loads of blood bags. Finally, it is imperative to know how long a device will keep its temperature in case of complete power failure.
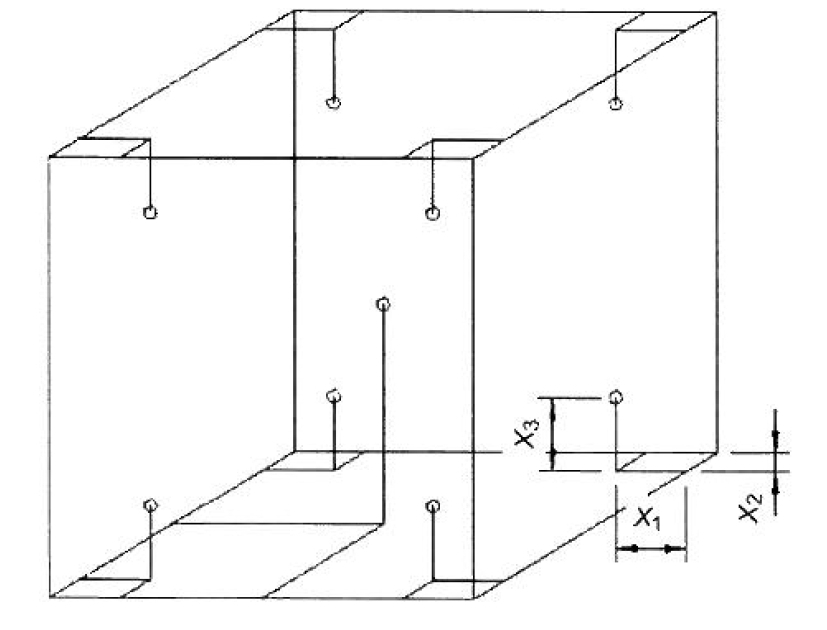
In order to perform such validation, a multi-sensor mapping of the device is performed. The number of sensors are dependent on a number of conditions and the size of the device or room to map. More details can be found in the International norm NEN-EN-IEC 60068-3-5:2018. Most commonly, all 8 corners of the device. The centre and the ambient temperature are recorded for a predetermined period of time (total of 9 positions). The results are then compared to the storage criteria of the blood bags or samples to be stored. If the device passes, it is fit for purpose. If it fails, it will need to be adjusted, repaired or replaced.
Temperature mapping is definitely not the only test that determines if a device is fit for purpose. Multiple parameters will determine if the samples can be stored In a device properly. Things like being able to recover quickly enough after a standardized door opening is one of them. Another common test is testing the “Hold Time” of a device with different loads. This time will determine, if the power fails, after how much time the samples/blood or medicines can no longer be used for patients or supplied to pharmacies or blood banks. Having a proper monitoring & alarm system in place also is a prerequisite for a quality management system.
For nearly 40 years, XiltriX has made ensuring the integrity and efficacy of a lab’s equipment, processes, and environment its sole focus. We help clients reduce staffing needs around calibration and validation while increasing both confidence and reporting around these processes.
CLICK ON THE THIS LINK TO DOWNLOAD THE PDF OF THE WHITE PAPER
Contact us to learn how we can help you speed and optimize these critical processes and much more.
Telephone: +31-73-5212229
Email: sales@xiltrix.com
Website: www.xiltrix.com
Han Weerdesteyn
CCO
January 2023
If you would like to know more about XiltriX, let me know.
Han Weerdesteyn
CCO