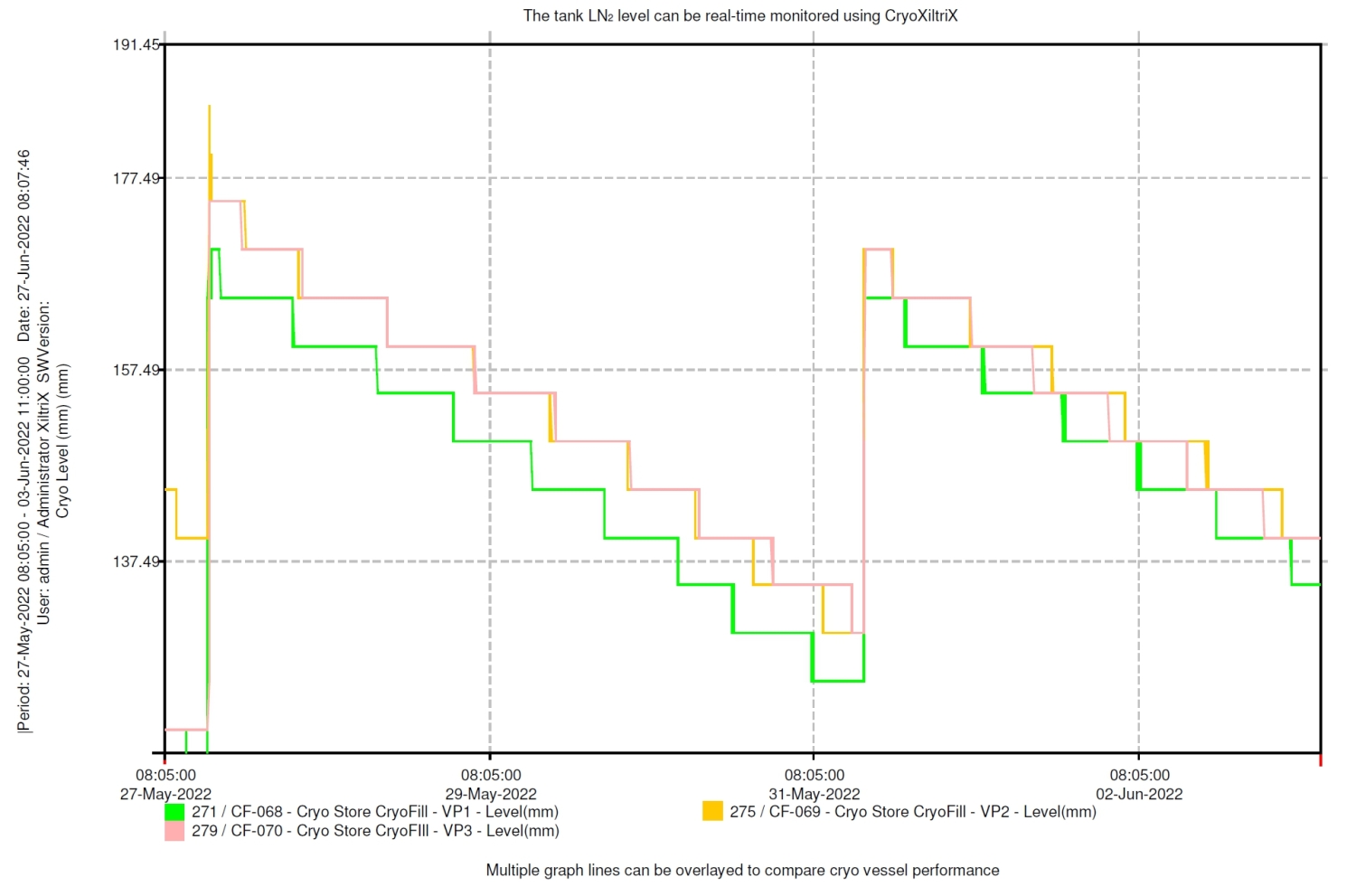
CryoFill Integration and Cryogenic Efficiency Optimisation in Hewitt Fertility Centre Liverpool
Future proofing a Cryogenic repository
In 2008 The Hewitt Fertility Centre moved into their new facility in the Liverpool Women’s Hospital. The brand-new facility was outfitted with state of the art automatically filling large cryogenic vessels and a facility wide XiltriX system. In the years to follow the lab had steadily grown with the number cryogenic samples and cycles increasing. Looking towards the future making sure the labs is ready for this whilst adhering to the strictest of quality procedures is a monumental
challenge.
The Story of Hewitt Fertility Centre
After being established as a branch of The Royal Liverpool Hospital and beginning to treat patients in 1989, the first baby born as a result of IVF treatment carried out at the IVF unit (which now we know as the Hewitt Fertility Centre) was delivered in 1990. Over time the facility grew and investment in new technologies were embraced. With the first ICSI baby in 1997, the Freezing of eggs in 2008, to offering Time lapse technologies to all patients from 2013 onwards, The Hewitt Fertility Centre has always been on the cutting edge of new developments in science.
This also goes for the embracing quality across the facility. As an (NHS) Trust member under scrutiny of the HFEA, frequent audits are a part of normal operations. Recognizing early on that quality monitoring was imperative, XiltriX was chosen as the centralised monitoring and alarm system as early as 2005. XiltriX has grown with the facility, including its expansion to the Knutsford site in 2013. Ms. Hannah Newby (Lead Clinical Embryologist) has been with the Hewitt Fertility Centre since 2005 and has witnessed its growth and development. Dr Rachel Gregoire (Scientific Director & HFEA person), with more than 20 years of experience in the field of Reproductive Science and Embryology, is tasked with managing the laboratories’ financial reports and scientific development.
Challenges overcome during implementation
The first and most difficult challenge of this project was to get a clear overview of all the requirements, with regards to liquid Nitrogen control, safety and storage expansion, LN₂ supply, automatic filling and monitoring integration, IT and building integration and all the other third party suppliers involved in the process. Only after this parts of the project would be completed could a comprehensive project plan be delivered and the necessary funds requested from the Trust. Since none on the Third party suppliers were able to supply the level of integration desired for this project, both XiltriX International and Cryo Products were asked to consult, still in the middle of the Pandemic. Ms. Newby commented: “As we had a lot of requirements for the upgrade of the cryogenic facility, the role of XiltriX and Cryo Products as solution providers has been imperative in making this project possible. By breaking down the challenges into manageable chunks a clear project plan with mile stones was delivered and offered to the NHS for sign off.”
Download
A full version of this article can be downloaded by THIS DOWNLOAD LINK. Or click the link next to this article.
If you would like to know more about XiltriX, let me know.
Han Weerdesteyn
CCO