 https://xiltrix.com/wp-content/uploads/2025/09/UZ-Brussel-CS4-scaled.jpg
1707
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2026-02-10 11:15:172026-02-10 11:20:25‘It means I can sleep at night’: how sensors are helping to solve scientists’ problems
https://xiltrix.com/wp-content/uploads/2025/09/UZ-Brussel-CS4-scaled.jpg
1707
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2026-02-10 11:15:172026-02-10 11:20:25‘It means I can sleep at night’: how sensors are helping to solve scientists’ problems
XiltriX offers real-time monitoring and alarm services for laboratory environments, reducing sample loss risks and damage to your science.
The system includes industrial grade hardware, redundant communication architecture, secure cloud data storage, 24/7 live agent technical support, and customizable alarm settings for specific conditions.
If you want to sleep sound at night without false alarms, let us contact you.
 https://xiltrix.com/wp-content/uploads/2025/09/UZ-Brussel-CS4-scaled.jpg
1707
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2026-02-10 11:15:172026-02-10 11:20:25‘It means I can sleep at night’: how sensors are helping to solve scientists’ problems
https://xiltrix.com/wp-content/uploads/2025/09/UZ-Brussel-CS4-scaled.jpg
1707
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2026-02-10 11:15:172026-02-10 11:20:25‘It means I can sleep at night’: how sensors are helping to solve scientists’ problems
XiltriX International Marketing Calendar 2026
The XiltriX International new Global Marketing Calendar 2026 is officially live. See where you can meet our team in the upcoming year.

XiltriX Monitoring-As-A-Service
XiltriX Monitoring as a Service is a transformation in how laboratories and facilities safeguard data, assets, and reputation.

Limited Edition Dr. Xil T-Rex Carnavals Pin
XiltriX International has designed a limited edition Dr Xil T-Rex pin to be worn on scarfs or jackets.

CryoXiltriX Integration at Cryoport Systems’ GMP Validated Site in Belgium
What makes this approach unique is that XiltriX can monitor and visualize the entire cryogenic ecosystem built by Cryo Solutions.

XiltriX International ISO 27001:2022 Certified
At XiltriX, the process was achieved within just twelve months, the company worked together to design, implement, and refine their ISMS.

Replacing Octax with XiltriX Service in KWZ an der Oper Munich
Octax was declared EOL, putting a lot of labs in a tough spot with regards to risk mitigation. The lab needed a new & well supported system.

Seamless Integration of Astec Incubators in XiltriX monitoring System
XiltriX collaborated with Astec Bio on dedicated connection cables, which are kept in stock at XiltriX for rapid deployment worldwide.
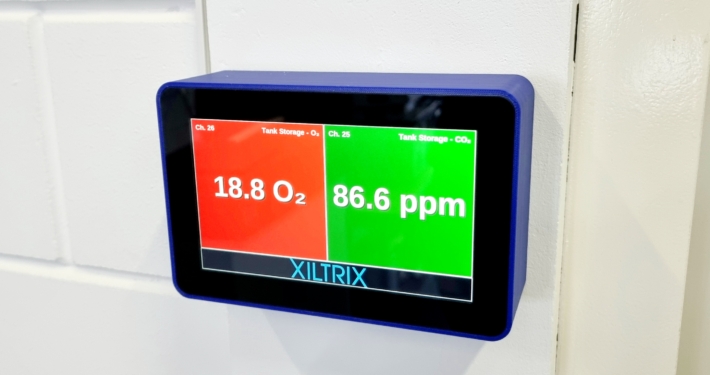
Enhancing Safety and Efficiency: XiltriX Visualization Screens in Cryo Rooms
By leveraging the power of the XiltriX visualization screen, facilities can transform safety and efficiency during LN₂ filling cycles.

5 Reasons Why Installing a Monitoring Sensor Through a Door Gasket Is a Bad Idea
Although it is tempting to quickly place a sensor behind a door gasket, the risks and disadvantages far outweigh any time saved.

Promo Market UMCG 2025: XiltriX as a Reliable Partner
On 11 Sep 2025, The UMCG was buzzing with activity during the annual Promo Market, XiltriX was exhibiting at.

15 Years XiltriX at Brussels IVF: Evolution of a Monitoring Partnership
A partner with one centralized monitoring platform, with the right system and service level, needed to be chosen.

The Critical Difference Between Data Loggers and Real-Time Data Acquisition Systems
Data Loggers and Real-time Data Acquisition Systems both aim to protect science, their methods and limitations differ greatly

White Paper – The Ultimate Guide to Dewar Monitoring and Alarm
Dewars are the standard for long term tissue storage in many laboratories. This is the Ultimate Guide to Dewar Monitoring and Alarm.

The Integration of XiltriX with Message Brokers
The integration of XiltriX with message brokers such as Messagebird and IQMessenger is an innovative and promising step forward

Effortless Visualization Screens with Real-time XiltriX Data
Showing the real-time value and alarm status of all relevant parameters is something that can be achieved utilizing the power of the XiltriX API.

XiltriX International – Team 2025!
In December 2024 we had the opportunity to bring our whole team together for the Christmas celebrations.

XiltriX International 2025 Marketing Calendar
As the holiday season is upon us, we are already planning ahead for a new year of meetings and trade shows. We would like to share our marketing calendar for the first half of 2025.

XiltriX International succesfully recertified NEN-EN-ISO 9001:2015
We are proud to report that the certification was successfully passed. Again, without a single non-conformity!

Case Study – Erasmus MC Central Biobank relies on XiltriX for Risk Mitigation
The Central Freezer Facility started in the Erasmus MC in Rotterdam started with 56 Nordic Freezers containing up to 164.640 samples.

Case Study – XiltriX partnering with IVF Group Ferty9 Fertility Center, India
The Ferty9 IVF group has taken the next steps in optimizing quality by installing XiltriX cloud monitoring in the Kukatpally lab, India.
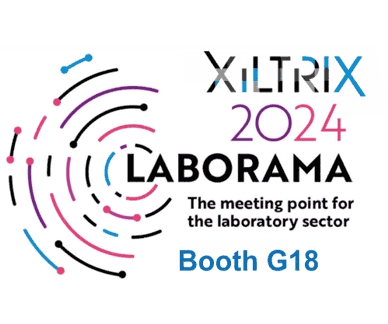
XiltriX at Laborama 2024 – Brussel Expo – Hall 3, Booth G18
XiltriX International is happy to join into the 24th edition of the Laborama, The main event for the laboratory industry, taking place on 14 & 15 March.
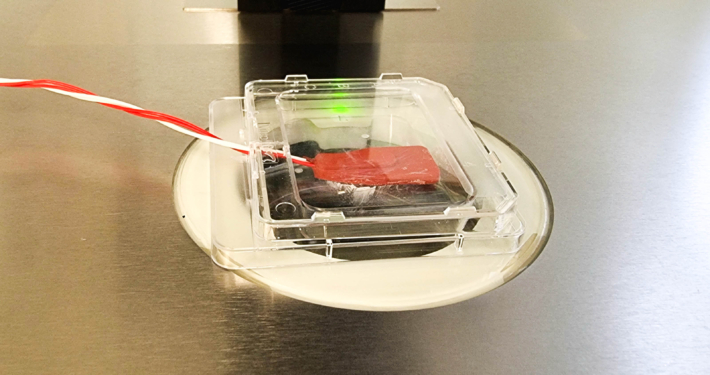
5 Pitfalls when Measuring Surface Temperatures (and how to fix them)
Measuring surface temperature in laboratories is not as easy as it sounds. Heated surfaces are frequently used to heat samples (even embryos), but can the display temperature of a heated surface actually be trusted?

XiltriX International 2024 Marketing Calendar
As the holiday season is upon us, we are already planning ahead for a new year of meetings and trade shows. We would like to share our marketing calendar for the first half of 2024.

Dr Xil T-Rex Holiday Photo Competition Winner 2023
Last summer Dr Xil T-Rex travelled the world. The XiltriX celebration comity was very interested in where the Dr went and asked his travel compagnons to make pictures of as many places as possible.

Case Study – XiltriX Monitoring Past to Present; Lessons Learned in 20 years, ETZ IVF Tilburg
The ETZ IVF laboratory replaced its low O₂ box type CO₂ incubators with Miri benchtops. XiltriX in use for for 20 years was also upgraded.

XiltriX International company meeting March 2023
As a company XiltriX International has experienced a rapid growth both nationally as well as internationally; time to come together.

XiltriX International organisation change announcement
XiltriX International has grown rapidly over the years. With an increase in its international customer base, XiltriX has become a global brand for monitoring services.

XiltriX International 2023 Marketing Calendar – UPDATE
Being able to meet people up close again made us realize the value of sharing thoughts and experiences. But time is moving fast! XiltriX has put together a new marketing calendar for 2023.

What’s the Difference Between Calibration and Validation in the Lab?
If you work in a lab you likely need calibration and validation of your equipment and processes. Although they may sound similar, they should not be confused with one another.

XiltriX International training week 2022 big success!
Not having been able to meet in person and to share knowledge with a lot of our partners because of Covid, we were very grateful to organize the International Training week for XiltriX this year

Press release – XiltriX commissioned Kuwait Central Blood Bank
Very recently, our XiltriX monitoring service was commissioned in the Kuwait Central Blood Bank (KCBB) to safeguard the precious blood and plasma products.
 https://xiltrix.com/wp-content/uploads/2022/06/Hewitt-4-scaled.jpg
1920
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2022-06-27 22:56:272025-08-20 23:09:03Case Study XiltriX – CryoFill Integration and Cryogenic Efficiency Optimisation in Hewitt Fertility Centre Liverpool
https://xiltrix.com/wp-content/uploads/2022/06/Hewitt-4-scaled.jpg
1920
2560
Han Weerdesteyn
https://xiltrix.com/wp-content/uploads/2021/09/Xiltrix-protecting-your-laboratory-science.png
Han Weerdesteyn2022-06-27 22:56:272025-08-20 23:09:03Case Study XiltriX – CryoFill Integration and Cryogenic Efficiency Optimisation in Hewitt Fertility Centre Liverpool
First of eight XiltriX installations ART Fertility India commissioned
XiltriX was already used successfully for many years in the Middle East; ART Fertility Clinics choose XiltriX as monitoring system.

Labvision reports about XiltriX and Cryo Products joint project in Brazil
In the most recent Labvision a nice piece was printed about the joint project of XiltriX International and Cryo Products. The installation has been running very well for a prolonged period of time and has recently been expanded with scales, weighing the liquid Nitrogen, with XiltriX real-time monitoring also successfully integrated.

Case Study – Successful integration of XiltriX in the Erasmus MC ATMP Laboratory
Monitoring of temperatures, CO₂, humidity, differential pressure and particle counting (Environmental Monitoring System). XiltriX was chosen as the system to provide the real-time monitoring and alarm services.

XiltriX International 2022 Marketing Calendar
The last years have been challenging for a lot of people. The pandemic has made meeting people face-to-face difficult sometimes even impossible. It's time to make up for that!

Case Study – Benefits of NDIR over TC CO₂ Sensors inside high humidity CO₂ incubators
Inside cell culture laboratories CO₂ incubators are one of the most important tools to grow living cells.
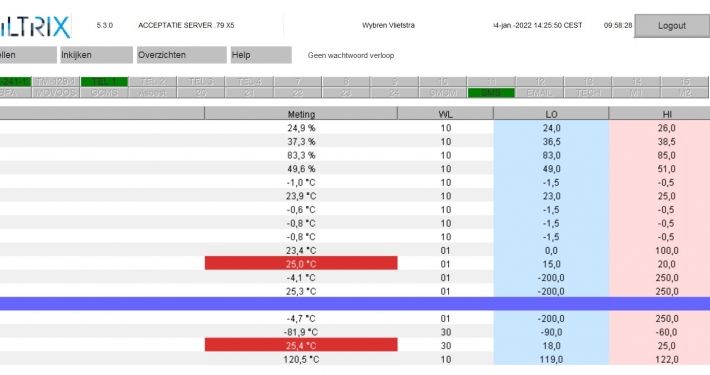
XiltriX launches Software V5.3
Major improvements in scalability and functionality. XiltriX International has just released it newest version of the widely used XiltriX software V5.3.

How XiltriX can make your life more convenient
Yesterday one of our customers, Dr. Samira B. Lima sent us a couple of pictures of her accessing XiltriX from her beach house in Brazil. The IT department had finalised the VPN setup so she could access XiltriX securely & remotely.

Security statement regarding: CVE-2021-44228, the log4j 2.x security vulnerability
Onlangs hebben meerdere internationale organisaties gebruikers gewaarschuwd voor een kwetsbaarheid in de log4j 2.x-software.

Successful Integration of CRYO360°™ Management System using CryoXiltriX in Sao Paulo Cryo Biorepository of CryoForLife
Between 2020 and 2021, CryoForLife projected and built a brand new Cryo360°™ cryo biorepository designed to store IVF and ART samples

XiltriX International gets NEN-EN-ISO 9001:2015 certification
Today we can officially announce that XiltriX International has received the certificate. I am really proud of the team for their hard work and enthusiasm!
Session 77: Driving Efficiencies in IVF Upwards
IVF laboratories are using more and more technology. Varying from benchtop incubators to automatically filled vapour phase storage tank. Not all of these devices can be connected into a centralized digital solution, making monitoring and alarming far from efficient.

Successful Implementation of XiltriX in the Dijklander IVF Lab
In early 2021 The Dijklander Hospital opened its new IVF laboratory to the public. XiltriX made sure they could sleep well at night and would be notified in the event of a calamity.

XiltriX Server & Communication Options; a solution for every problem
One of the quickest changing industries in recent years has been the world of IT. Instead of an evolution, there has been a revolution. Ever faster networks and internet connections have allowed for cloud applications which anyone with a smart phone can easily access. Having an easily accessible cloud software solution for a system like XiltriX is definitely nice, but there are a number of challenges not so easily overcome.

XiltriX Monitoring in IITA Nigeria
“In general, the XiltriX monitoring system was essential in the implementation of IITA cryobank, started in August 2017. Since the XiltriX International B.V. system was supplied and installed in 2016 at the IITA Genetic Resources Centre (GRC), it showed effectiveness, stability and easy handling for the inventory and monitoring of IITA cryobank. It has been in constant use and has functioned effectively, meeting all expected purposes.”

OOO: How to Use Data to Improve Operations
COVID-19 has put a lot off pressure on clinics to continue to function without the certainty of being able to go on site in case of an emergency.

XiltriX temperature and alarm monitoring of Esco Miri
XiltriX has been the monitoring system of choice for many laboratories for many years. Over the years XiltriX has developed bespoke solutions for the IVF industry to safeguard the precious samples of new life.

Anne van Veggel finished research internship
Today our intern Ms. Anne van Veggel finished her research internship on the possible integration of XiltriX with other data sources by handing in her research report.

Continued support by XiltriX International COVID-19 / Corona
In recent days, following the COVID-19 crisis, the Dutch government has taken far-reaching measures that have a major impact on our employees, customers and business operations. XiltriX International follows these advices as closely as possible, with the safety of employees and customers at the center.

XiltriX International at ESHRE in Vienna, June 23-26, 2019
XiltriX International will be attending the 35th Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE, https://www.eshre.eu/ESHRE2019) which will be held this year in Vienna, Austria, on June 23-26, 2019 at the MESSE WIEN Exhibition & Congress Center.

IKS International changes name to XiltriX
We are excited to share important news regarding the evolution of our company. We are delighted to announce that we will be changing our name to XiltriX. This will take place as of January 1, 2019.

XiltriX with CryoFill integration provides control to Boston Place IVF clinic, London UK
XiltriX with CryoFill integration provides control to Boston Place IVF clinic, London UK

XiltriX joins the affiliate program at the UC San Diego Center for Microbiome Innovation
San Diego, California, February 21, 2018 – XiltriX, a service that provides data acquisition, analysis, reporting and documentation for compliance and validation, has joined the UC San Diego Center for Microbiome Innovation (CMI) as an industry affiliate. XiltriX measures lab equipment, such as fridges, freezers and other vital equipment to ensure that it is functioning properly, across campus, and in real-time.
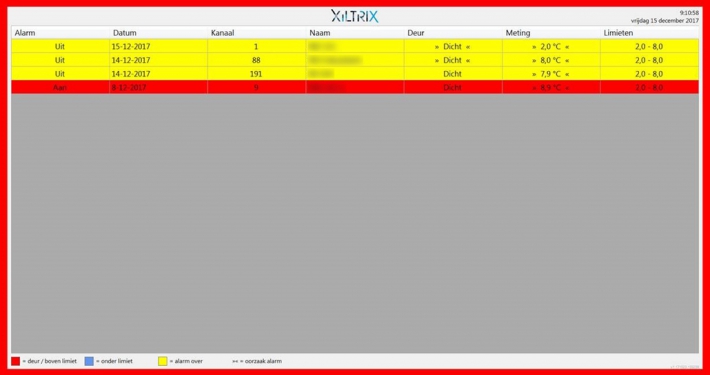
XiltriX software integration in AMC teaching hospital Amsterdam
XiltriX alarm integration provides new viewing functionality in Operating Theatre – XiltriX Email alarms are automatically processed on big television screens

Bio Specimen Research Symposium 27th & 28th of February 2018 in Luxembourg
IKS International will be present at the Bio Specimen Research Symposium in Luxembourg and we would like to invite you to visit us at booth #15 between the 27th and 28th of February 2018.

XiltriX GMP validated real-time monitoring system installed on 5 Eurofins sites across the Netherlands
ROSMALEN – Dec 21, 2017 – The company Eurofins Microsafe has been a user of the XiltriX monitoring and alarm system for nearly a decade. XiltriX was installed after the previous system did not live up to the expectations of the customer. XiltriX monitors the temperature in freezers, fridges and rooms, but also the temperature and CO2 level in incubators. XiltriX also monitors the differential pressure and particle counts in the cleanrooms and is calibrated and validated according to 21 CFR part 11 and GMP. After many years of use, we asked Mr. Tony van Weert, facilities engineer responsible for XiltriX, to describe his experiences with XiltriX over the years. He stated the following:

XiltriX real-time monitoring solution operating seamlessly for 2 years at Portland Oregon IVF laboratory
XiltriX instrumental in controlling the Oregon Reproductive Medicine IVF laboratory parameters; keeping gametes and embryos safe at all times

XiltriX launches its new substation Tethys
The XiltriX real-time solution has used the combination of a Calypso with the hard-wired Titan and the wireless Telesto substations for a number of years now.

XiltriX at ESHRE 2017 Geneva
The new XiltriX Saturn monitoring platform was launched at the show.

IKS International exhibiting at ESHRE 2017 Geneva
IKS International will be exhibiting our Realtime monitoring system XiltriX at booth D16.

XiltriX at ESHRE 2016 in Helsinki
Our team had the pleasure of welcoming our current distributors as well as having the opportunity to meet new partners and end-users who were excited about the implementation of XiltriX, our independent real-time monitoring system in their own laboratories.



















































